Herpes simplex virus type 2 (HSV-2) infections frequently present with a constellation of symptoms that extend beyond the characteristic genital lesions. Among the most significant yet often overlooked manifestations is inguinal lymphadenopathy, where lymph nodes in the groin region become enlarged and tender during both primary and recurrent episodes. This lymphatic response represents the body’s natural immune defence mechanism against viral invasion, yet it can cause considerable discomfort and anxiety for patients who may not understand the connection between their genital herpes diagnosis and swollen groin nodes.
The relationship between HSV-2 and lymphatic system involvement is particularly pronounced during initial infections, when the immune system encounters the virus for the first time. Understanding this pathophysiological connection is crucial for healthcare providers and patients alike, as it influences both diagnostic approaches and treatment strategies. The complexity of HSV-2’s interaction with the lymphatic system also necessitates careful differential diagnosis, as numerous other conditions can present with similar inguinal lymphadenopathy patterns.
HSV-2 pathophysiology and lymphatic system involvement
The pathophysiology of HSV-2 infection involves a complex cascade of viral replication and host immune responses that directly impact the regional lymphatic system. When HSV-2 initially breaches the genital mucosal barriers, it encounters a sophisticated network of immune cells and lymphatic vessels designed to contain and eliminate foreign pathogens. This initial confrontation sets in motion a series of events that culminate in the characteristic lymphadenopathy observed in many patients.
Herpes simplex virus type 2 replication mechanisms in genital tissues
HSV-2 demonstrates remarkable tropism for stratified squamous epithelium found in genital tissues, where it begins its replicative cycle through membrane fusion and viral entry. The virus employs glycoproteins gB, gD, gH, and gL to facilitate attachment and penetration into host cells, initiating a lytic infection that rapidly spreads to adjacent epithelial cells. During this acute phase, extensive viral replication occurs within the infected tissues, releasing numerous viral particles and cellular debris that serve as antigens for immune system recognition.
The viral replication process triggers significant inflammation in the affected genital tissues, leading to the characteristic vesicular lesions and ulcerations. Infected cells undergo cytolysis, releasing viral proteins and nucleic acids that are rapidly recognised as foreign by local antigen-presenting cells. This inflammatory response creates a chemotactic gradient that attracts various immune cells to the infection site, including dendritic cells, macrophages, and neutrophils that will ultimately carry viral antigens to regional lymph nodes.
Viral neurotropism and dorsal root ganglia latency establishment
Following initial replication in epithelial cells, HSV-2 demonstrates its characteristic neurotropism by travelling along sensory nerve fibres to establish latency in the dorsal root ganglia. This retrograde transport occurs via the axoplasmic flow mechanism, where viral nucleocapsids are transported along microtubules to reach the sacral ganglia, particularly S2-S4. The establishment of latency represents a critical phase in HSV-2 pathophysiology, as it ensures viral persistence despite immune clearance of the primary infection.
During the neurotropic phase, viral antigens continue to be processed and presented to the immune system, maintaining the inflammatory response that contributes to ongoing lymphadenopathy. The periodic reactivation of latent virus from dorsal root ganglia results in recurrent episodes of viral shedding and lymphatic activation. This cyclical pattern explains why some patients experience lymph node swelling even during minor recurrences that may not produce visible lesions.
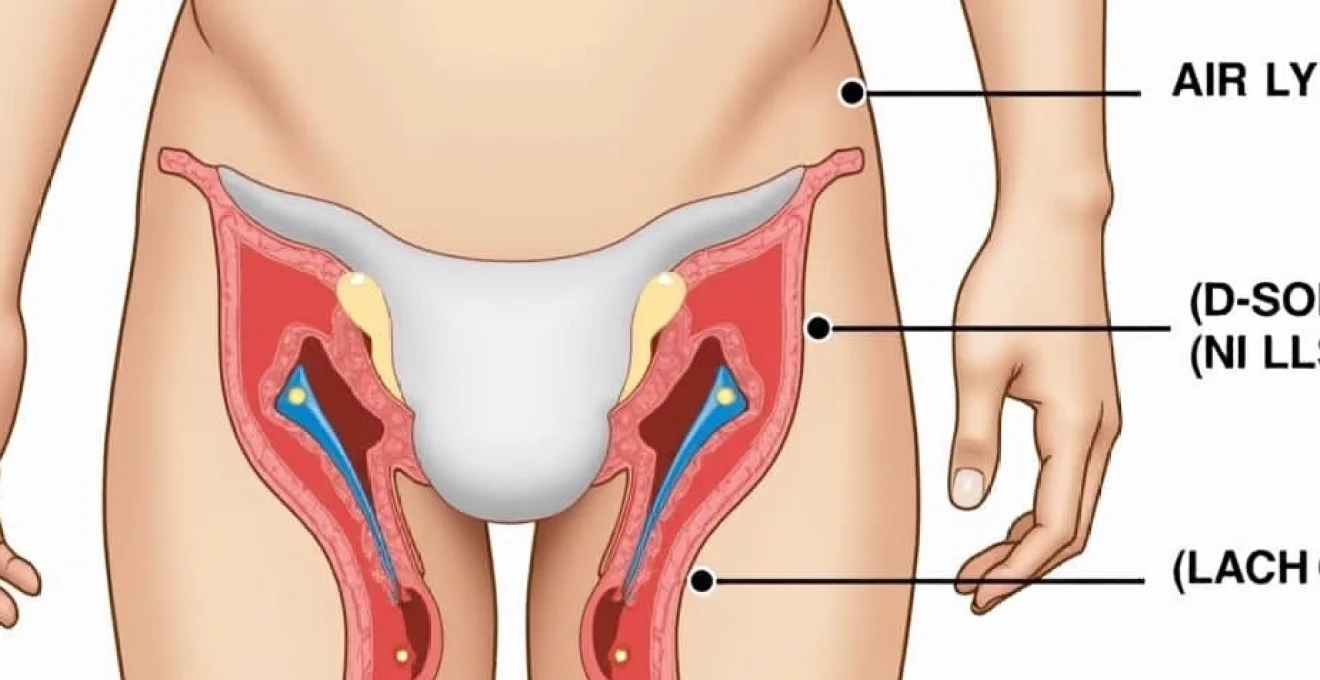
Lymphatic drainage pathways from genital region to inguinal nodes
The genital region possesses an extensive lymphatic drainage network that channels interstitial fluid and immune cells toward the inguinal and femoral lymph node chains. The superficial inguinal lymph nodes receive drainage from the external genitalia, perineum, and lower anal canal, while the deep inguinal nodes process lymph from deeper pelvic structures. This anatomical arrangement explains why HSV-2 infections consistently affect the groin lymph nodes rather than other regional node groups.
Lymphatic vessels from infected genital tissues become dilated and hyperactive during HSV-2 episodes, facilitating the transport of viral antigens, immune complexes, and activated immune cells to the regional nodes. The lymphatic endothelium itself may become infected with HSV-2, contributing to the inflammatory process and potentially serving as a reservoir for viral persistence. Understanding these drainage patterns is essential for clinicians assessing patients with suspected HSV-2-related lymphadenopathy.
Immune response cascade triggering lymphadenopathy
The development of inguinal lymphadenopathy during HSV-2 infection results from a complex immune response cascade initiated by viral recognition and antigen presentation. Dendritic cells and macrophages in the infected genital tissues phagocytose viral particles and infected cell debris, processing these antigens for presentation to T lymphocytes in the regional lymph nodes. This antigen presentation process triggers rapid lymphocyte proliferation and activation, leading to node enlargement.
The inflammatory cascade involves multiple cytokines and chemokines, including interferon-α, interleukin-1β, tumour necrosis factor-α, and various chemokines that recruit additional immune cells to the lymph nodes. B lymphocytes undergo clonal expansion and differentiation into plasma cells, producing HSV-2-specific antibodies. Simultaneously, CD4+ and CD8+ T cells proliferate and differentiate into effector cells capable of viral clearance. This intense immune activity results in the characteristic tender, enlarged lymph nodes observed clinically.
Clinical manifestations of HSV-2-Associated inguinal lymphadenopathy
The clinical presentation of HSV-2-associated inguinal lymphadenopathy varies considerably between primary and recurrent infections, with primary episodes typically producing more pronounced and prolonged lymph node involvement. Patients often report tender, enlarged nodes that develop concurrently with or shortly after the appearance of genital lesions. The lymphadenopathy may persist for several weeks, often outlasting the visible genital lesions, which can be confusing for patients who expect all symptoms to resolve simultaneously.
Bilateral versus unilateral inguinal node enlargement patterns
HSV-2 infections commonly produce bilateral inguinal lymphadenopathy, particularly during primary episodes when viral load and immune activation are at their peak. The bilateral pattern reflects the extensive lymphatic drainage network of the genital region and the tendency for HSV-2 to affect multiple sites during initial infection. However, unilateral lymphadenopathy can occur when the infection is localised to one side of the genital tract or during limited recurrent episodes.
The pattern of lymph node involvement often correlates with the distribution of genital lesions, though this relationship is not absolute. Infections affecting the vulva or penile shaft typically produce more pronounced superficial inguinal node enlargement, while infections involving deeper structures may primarily affect the deep inguinal or external iliac nodes. Clinicians should palpate both superficial and deep node groups to obtain a complete assessment of lymphatic involvement.
Temporal relationship between primary outbreak and lymph node swelling
The temporal relationship between HSV-2 lesions and lymphadenopathy follows a predictable pattern during primary infections, with lymph node swelling typically appearing 2-7 days after the onset of genital symptoms. The lymphadenopathy often reaches its peak size and tenderness during the second week of the primary episode, coinciding with maximal immune system activation. This timing reflects the period required for antigen processing, transport to lymph nodes, and subsequent immune cell proliferation.
During recurrent episodes, the temporal relationship may be less pronounced or even reversed, with some patients reporting lymph node tenderness preceding visible lesions by 1-2 days. This phenomenon, known as prodromal lymphadenopathy, likely reflects the more rapid immune response that occurs during reactivation episodes. The lymph node involvement during recurrences is typically milder and shorter-lived than during primary infections, usually resolving within a few days to one week.
Palpable node characteristics: size, consistency, and mobility assessment
HSV-2-associated lymph nodes typically measure 1-3 centimetres in diameter and exhibit characteristic physical findings that help differentiate them from nodes enlarged due to other causes. The nodes are usually firm but not hard, mobile rather than fixed to underlying tissues, and distinctly tender to palpation. The overlying skin remains normal in appearance without erythema or warmth, distinguishing HSV-2 lymphadenopathy from bacterial lymphangitis or abscess formation.
The consistency of HSV-2-related lymph nodes reflects the intense lymphoid hyperplasia occurring within the node architecture. Unlike malignant nodes, which tend to be hard and fixed, or bacterial infections, which may be fluctuant if abscess formation occurs, HSV-2 nodes maintain their rubbery consistency throughout the episode. The mobility of these nodes indicates the absence of significant periadenitis or invasion of surrounding structures, features that would suggest alternative diagnoses.
Associated systemic symptoms during acute HSV-2 episodes
Primary HSV-2 infections frequently produce significant systemic symptoms that accompany the local genital manifestations and inguinal lymphadenopathy. These constitutional symptoms include fever, which may reach 38-39°C, generalised malaise, myalgia, and headache. The systemic symptom complex typically develops 1-2 days after the appearance of genital lesions and may persist for 3-7 days during primary episodes.
The severity of systemic symptoms often correlates with the degree of lymphadenopathy, as both reflect the intensity of the immune response to the primary HSV-2 infection. Some patients also experience urinary retention or dysuria due to local inflammation and autonomic nervous system involvement. These systemic manifestations are less common and milder during recurrent episodes, reflecting the more limited viral replication and immune activation that occurs during reactivation.
Differential diagnosis of groin lymphadenopathy in HSV-2 context
Establishing an accurate diagnosis of HSV-2-associated lymphadenopathy requires careful consideration of numerous alternative conditions that can produce similar clinical presentations. The differential diagnosis encompasses infectious, inflammatory, and neoplastic conditions, each with distinct epidemiological, clinical, and laboratory characteristics. A systematic approach to differential diagnosis is essential, as treatment strategies and prognoses vary significantly between these conditions.
Distinguishing HSV-2 from treponema pallidum lymphadenitis
Syphilis, caused by Treponema pallidum , represents one of the most important differential diagnoses for HSV-2-associated lymphadenopathy, particularly during primary syphilis when chancres and regional lymphadenopathy develop concurrently. Primary syphilitic chancres typically present as painless, indurated ulcers with clean bases and raised borders, contrasting sharply with the painful, shallow ulcerations characteristic of HSV-2. The lymphadenopathy associated with primary syphilis is characteristically non-tender, firm, and rubbery, differing from the tender nodes typical of HSV-2 infection.
Laboratory testing plays a crucial role in distinguishing these conditions, as dark-field microscopy can identify spirochetes in chancre exudate, while serological tests including RPR, VDRL, and treponemal-specific assays confirm syphilis diagnosis. The temporal evolution also differs, with syphilitic chancres typically healing spontaneously within 3-6 weeks regardless of treatment, while HSV-2 lesions may persist or recur. Co-infection with both pathogens can occur, necessitating testing for both conditions in sexually active individuals presenting with genital ulceration and lymphadenopathy.
Chlamydia trachomatis LGV serovars L1-L3 comparison
Lymphogranuloma venereum (LGV), caused by Chlamydia trachomatis serovars L1, L2, and L3, produces a distinctive pattern of genital ulceration followed by significant inguinal and femoral lymphadenopathy. The primary lesion in LGV is typically a small, painless papule or shallow ulcer that often goes unnoticed by patients. The subsequent development of massive, matted lymphadenopathy occurring 2-6 weeks later represents the characteristic secondary stage of LGV, often with the pathognomonic “groove sign” where enlarged inguinal and femoral nodes are separated by the inguinal ligament.
LGV lymphadenopathy tends to be more extensive and persistent than HSV-2-associated nodes, often progressing to suppuration and fistula formation without appropriate treatment. The nodes in LGV may become fluctuant and eventually rupture, creating chronic draining sinuses. Microimmunofluorescence testing and nucleic acid amplification tests can definitively diagnose LGV, while the clinical course of progressive lymphadenopathy over weeks to months helps differentiate it from the more acute presentation of HSV-2.
Behçet’s disease and inflammatory bowel disease lymph node involvement
Behçet’s disease, a chronic multisystem vasculitis, can produce recurrent genital ulceration and associated lymphadenopathy that may be confused with HSV-2 infection. Behçet’s genital ulcers are typically deeper and more persistent than HSV-2 lesions, often leaving significant scarring upon healing. The lymphadenopathy in Behçet’s disease may be part of a more generalised lymphatic involvement and is often accompanied by other systemic manifestations including oral ulcers, ocular inflammation, skin lesions, and arthritis.
Inflammatory bowel diseases, particularly Crohn’s disease, can occasionally present with genital ulceration and regional lymphadenopathy, especially when involving the perianal region. These ulcers tend to be linear, deep, and associated with significant tissue oedema and inflammation. The lymphadenopathy is typically less prominent than in infectious causes and may be accompanied by gastrointestinal symptoms, extraintestinal manifestations, and characteristic endoscopic and histological findings. The chronic, indolent nature of these conditions contrasts with the episodic pattern typical of HSV-2 infections.
Malignant lymphoproliferative disorders presenting with inguinal masses
Malignant lymphoproliferative disorders, including Hodgkin lymphoma, non-Hodgkin lymphomas, and metastatic carcinomas, can present with inguinal lymphadenopathy that must be differentiated from benign infectious causes like HSV-2. Malignant nodes typically exhibit certain characteristic features including firm to hard consistency, fixed attachment to underlying structures, non-tender nature, and progressive enlargement over weeks to months. The absence of associated genital lesions or systemic infectious symptoms should raise suspicion for malignant causes.
Primary genital malignancies, including squamous cell carcinoma, melanoma, and rare sarcomas, may present with both local lesions and metastatic inguinal lymphadenopathy. These lesions typically appear as persistent, non-healing ulcers or masses that fail to respond to antiviral therapy. The lymph nodes in metastatic disease are characteristically hard, fixed, and may be matted together. Biopsy of suspicious nodes and primary lesions is essential for definitive diagnosis, particularly when clinical features suggest malignancy or when empirical antimicrobial therapy fails to produce improvement.
Laboratory diagnostics and PCR testing protocols
Accurate laboratory diagnosis of HSV-2 infection and its associated lymphadenopathy relies on multiple diagnostic modalities, each with specific indications, advantages, and limitations. Polymerase chain reaction (PCR) testing has emerged as the gold standard for HSV diagnosis due to its superior sensitivity and specificity compared to traditional viral culture methods. The choice of diagnostic test depends on the clinical presentation, timing of sample collection, and available laboratory resources.
During acute episodes with visible lesions, PCR testing of vesicle fluid or ulcer swabs provides the highest diagnostic yield, with sensitivity rates exceeding 95% for detecting HSV DNA. The ability of PCR to differentiate between HSV-1 and HSV-2 is crucial for prognosis and counselling, as HSV-2 genital infections typically recur more frequently than HSV-1 genital infections. Real-time PCR platforms can provide results within hours, facilitating rapid clinical decision-making and immediate initiation of appropriate antiviral therapy.
Type-specific serological testing serves as an important complementary diagnostic tool, particularly for patients presenting with lymphadenopathy but no visible lesions, or when determining infection status in asymptomatic individuals. HSV-2 IgM antibodies typically appear 1-2 weeks after initial infection, while IgG antibodies develop 2-4 weeks post-infection and persist lifelong. Western blot testing remains the confirmatory standard for equivocal serological results, though newer enzyme immunoassays demonstrate improved accuracy in differentiating HSV-1 from HSV-2 antibodies.
For patients presenting with isolated inguinal lymphadenopathy without obvious genital lesions, fine needle aspiration of enlarged nodes may be considered when PCR testing of potential lesions is negative. Lymph node aspirates can be tested for HSV DNA using PCR techniques, though this approach is reserved for cases where clinical suspicion remains high despite negative conventional testing. The timing of sample collection is crucial, as viral DNA levels decline rapidly after lesion healing, potentially leading to false-negative results if testing is delayed.
Treatment protocols for HSV-2 with concurrent lymphadenopathy
The management of HSV-2 infections complicated by inguinal lymphadenopathy requires a comprehensive approach addressing both viral suppression and symptom control. Antiviral therapy remains the cornerstone of treatment, with nucleoside analogues demonstrating proven efficacy in reducing viral replication, accelerating lesion healing, and potentially diminishing the intensity of associated lymphadenopathy. The choice between episodic and suppressive therapy depends on infection frequency, severity of symptoms, and patient preference.
For primary HSV-2 episodes with significant lymphadenopathy, oral aciclovir 400mg three times daily for 7-10 days represents first-line therapy, though valaciclovir 1g twice daily or famciclovir 250mg three times daily offer improved bioavailability and convenience. Severe primary infections with extensive lymphadenopathy may require hospitalisation and intravenous aciclovir, particularly when complications such as urinary retention, secondary bacterial infection, or disseminated disease are present. The antiviral therapy should be initiated as early as possible, ideally within 72 hours of symptom onset, to maximise therapeutic benefit.
Suppressive antiviral therapy should be considered for patients experiencing frequent recurrent episodes with troublesome lymphadenopathy, typically defined as six or more episodes annually. Daily suppressive regimens using aciclovir 400mg twice daily, valaciclovir 500-1000mg once daily, or famciclovir 250mg twice daily can reduce recurrence frequency by 70-80% and may diminish the severity of associated lymph node involvement. Regular monitoring of renal function is recommended for patients on long-term suppressive therapy, particularly those with pre-existing kidney disease or concurrent nephrotoxic medications.
Symptomatic management of tender inguinal lymphadenopathy includes topical and systemic analgesics, with non-steroidal anti-inflammatory drugs (NSAIDs) providing both pain relief and anti-inflammatory effects. Warm compresses applied to enlarged nodes may offer additional comfort, though care must be taken to avoid skin maceration in the presence of active genital lesions. Some patients benefit from loose-fitting clothing and avoidance of activities that place pressure on affected lymph nodes during acute episodes.
Long-term prognosis and recurrence prevention strategies
The long-term prognosis for patients with HSV-2-associated inguinal lymphadenopathy is generally excellent, with most individuals experiencing a gradual reduction in both recurrence frequency and severity over time. Natural history studies demonstrate that lymphadenopathy becomes less prominent with subsequent episodes, as the immune system develops more efficient viral control mechanisms. However, the psychological impact of recurrent symptoms, including lymph node swelling, can significantly affect quality of life and sexual relationships.
Recurrence prevention strategies encompass both pharmacological and lifestyle modifications aimed at reducing viral reactivation triggers. Stress management techniques, including regular exercise, adequate sleep, and psychological counselling when appropriate, play crucial roles in maintaining immune system stability. Patients should be educated about common triggers such as illness, immunosuppression, trauma, and hormonal fluctuations, enabling proactive management of high-risk periods.
Nutritional support focusing on immune system enhancement may provide additional benefits, with evidence suggesting that L-lysine supplementation, zinc, and vitamin C may help reduce recurrence frequency in some individuals. However, these interventions should complement rather than replace proven antiviral therapies. Patient education regarding transmission risk reduction, including condom use, disclosure to sexual partners, and recognition of prodromal symptoms, remains essential for comprehensive management.
Regular follow-up care should address both physical and psychological aspects of HSV-2 infection, with particular attention to monitoring for complications such as secondary bacterial infection of lymph nodes, development of chronic pain syndromes, or depression related to diagnosis. Healthcare providers should maintain open communication about treatment options, as new antiviral agents and therapeutic approaches continue to emerge. The integration of telemedicine platforms has improved access to specialised care, enabling more frequent monitoring and adjustment of treatment regimens based on individual patient responses and changing clinical circumstances.