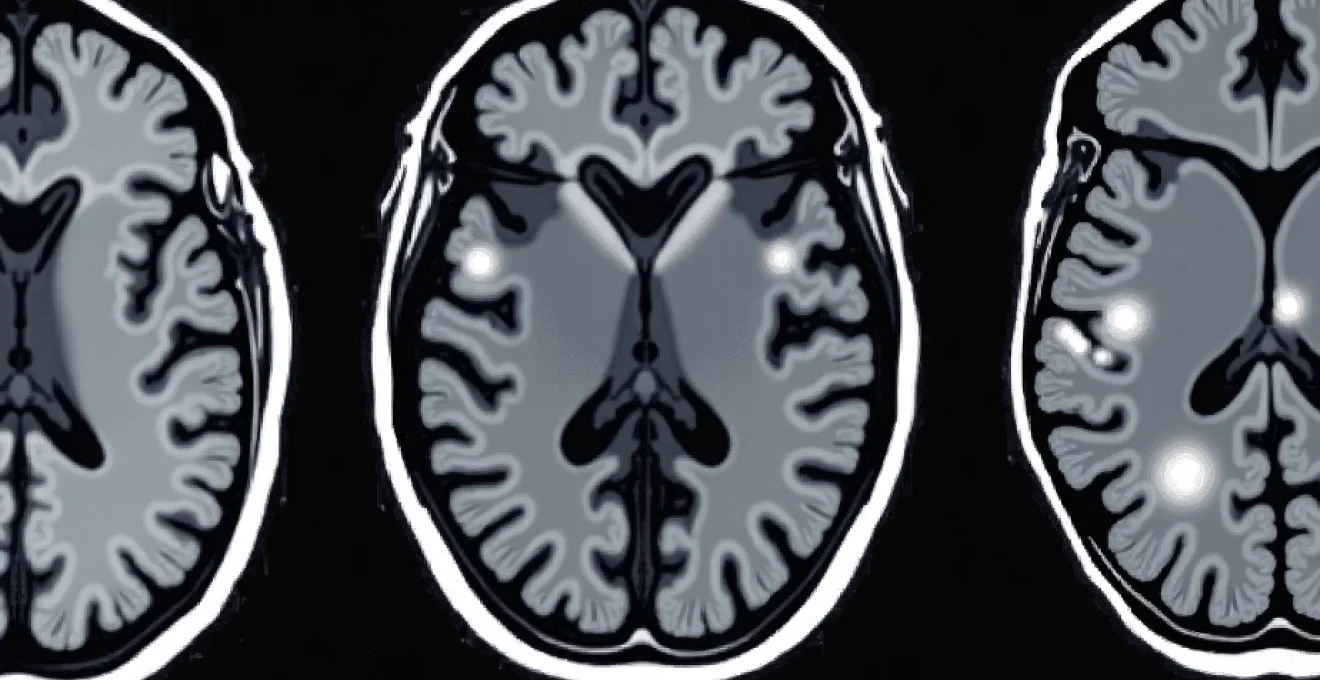
White spots appearing on brain MRI scans represent one of the most frequently encountered radiological findings in modern neuroimaging, yet they can cause considerable anxiety for patients and present diagnostic challenges for clinicians. These hyperintense signals, technically known as white matter hyperintensities, appear as bright areas on specific MRI sequences and can indicate a wide spectrum of conditions ranging from normal ageing changes to serious neurological diseases. Understanding the clinical significance of these findings requires careful analysis of their location, pattern, and association with patient symptoms and medical history.
The interpretation of white matter lesions has evolved significantly with advances in MRI technology, allowing radiologists to detect increasingly subtle changes in brain tissue. However, this enhanced sensitivity has also highlighted the complexity of distinguishing between benign age-related changes and pathological processes that require medical intervention. The presence of white spots on brain MRI doesn’t automatically indicate disease, but their characteristics can provide valuable insights into underlying vascular health, inflammatory conditions, and neurodegenerative processes.
Understanding hyperintense T2 and FLAIR signal abnormalities on brain MRI scans
White matter hyperintensities manifest as bright signal abnormalities on T2-weighted and fluid-attenuated inversion recovery (FLAIR) MRI sequences. These imaging techniques are particularly sensitive to changes in tissue water content and can detect subtle alterations in brain microstructure that may not be visible on other sequences. The appearance of these lesions reflects disruption of normal tissue architecture, often involving damage to myelin sheaths, axonal integrity, or changes in tissue hydration.
The pathophysiology underlying these signal changes typically involves compromise to the blood-brain barrier, inflammatory processes, or ischaemic damage to white matter tracts. When myelin becomes damaged or degraded, the affected tissue exhibits increased water content, which translates to hyperintense signal on T2 and FLAIR sequences. This process can occur gradually over months or years, or may develop more rapidly in acute inflammatory conditions.
T2-weighted and FLAIR sequence technical parameters for white matter lesion detection
FLAIR sequences prove particularly valuable for detecting periventricular white matter changes because they suppress the bright signal from cerebrospinal fluid, allowing better visualisation of lesions adjacent to the ventricular system. Modern 3T MRI scanners can achieve submillimetre resolution, enabling detection of lesions as small as 2-3 millimetres in diameter. The technical parameters used, including echo time, repetition time, and slice thickness, significantly influence lesion conspicuity and detection sensitivity.
Periventricular white matter hyperintensities: Location-Specific diagnostic significance
Periventricular white matter lesions occur in the brain tissue immediately surrounding the lateral ventricles and represent a particularly important subset of white matter changes. These lesions often reflect small vessel disease affecting the deep penetrating arteries that supply this vulnerable watershed region. The periventricular location is significant because it’s an area prone to ischaemic damage due to its position at the border zones between different vascular territories.
The clinical significance of periventricular lesions varies considerably depending on their extent and associated findings . While small, punctate periventricular hyperintensities are common in elderly individuals and may represent normal ageing, extensive confluent changes can indicate more significant vascular pathology requiring further investigation and potential treatment.
Deep white matter lesions: subcortical distribution patterns and clinical correlations
Deep white matter lesions occur within the subcortical regions and typically affect the long association fibres connecting different cortical areas. These lesions often correlate with cognitive impairment, particularly affecting executive function, processing speed, and working memory. The subcortical location makes these lesions particularly disruptive to neural networks, as they can disconnect critical brain regions involved in cognitive processing.
Research has demonstrated that the volume and confluency of deep white matter lesions correlate more strongly with cognitive decline than periventricular changes. This relationship likely reflects the involvement of important white matter tracts that facilitate communication between frontal and posterior brain regions essential for complex cognitive tasks.
Juxtacortical white matter signal changes: Grey-White matter junction abnormalities
Juxtacortical lesions occur at the interface between grey and white matter, representing a distinct category of white matter changes with specific diagnostic implications. These lesions are particularly significant in the context of multiple sclerosis, where they represent one of the dissemination criteria used for diagnosis. The juxtacortical location suggests involvement of U-fibres, the short association pathways connecting adjacent cortical regions.
Multiple sclerosis demyelinating lesions: McDonald criteria and radiological patterns
Multiple sclerosis represents one of the most important causes of white matter lesions in younger adults, characterised by inflammatory demyelinating plaques distributed throughout the central nervous system. The radiological diagnosis of MS relies heavily on the McDonald criteria, which specify particular patterns of lesion distribution and timing that help distinguish MS from other conditions causing white matter abnormalities.
MS lesions typically demonstrate specific characteristics that aid in diagnosis: they tend to be ovoid in shape, show perpendicular orientation to the ventricular surface, and often exhibit gadolinium enhancement during active phases. The distribution pattern is crucial, with involvement of periventricular, juxtacortical, infratentorial, and spinal cord regions required to meet diagnostic criteria for dissemination in space.
Dawson’s fingers: pathognomonic periventricular lesion morphology in MS
Dawson’s fingers represent a highly characteristic radiological sign in multiple sclerosis, consisting of periventricular lesions that extend perpendicularly from the ventricular surface in a finger-like pattern. These lesions follow the distribution of periventricular veins and reflect the perivenous inflammatory pattern typical of MS pathology. The recognition of Dawson’s fingers can be virtually pathognomonic for multiple sclerosis , particularly when seen in conjunction with other typical MS features.
This distinctive morphology results from inflammation surrounding the deep medullary veins, creating elongated lesions that extend radially from the ventricular surface into the adjacent white matter. Advanced imaging techniques, including susceptibility-weighted imaging, can sometimes demonstrate the central vein within these lesions, further supporting the MS diagnosis.
Corpus callosum lesions: interhemispheric white matter tract involvement
Corpus callosum lesions represent another important feature in MS diagnosis and are included in the periventricular lesion category within the McDonald criteria. These lesions typically occur at the undersurface of the corpus callosum, following the distribution of pericallosal veins. The callosal involvement is significant because it represents the largest interhemispheric white matter tract, and lesions in this location can cause interhemispheric disconnection syndromes.
Brainstem and cerebellar peduncle demyelinating plaques
Infratentorial lesions affecting the brainstem and cerebellar peduncles contribute to the dissemination in space criteria for MS diagnosis. These lesions often produce distinctive clinical syndromes, including internuclear ophthalmoplegia, diplopia, ataxia, and cranial nerve palsies. The brainstem location is particularly significant because even small lesions can cause prominent neurological symptoms due to the high density of neural pathways in this region.
Gadolinium enhancement patterns: active versus chronic MS lesions
Gadolinium enhancement patterns provide crucial information about disease activity in multiple sclerosis, distinguishing between active inflammatory lesions and chronic inactive plaques. Active lesions typically show ring enhancement or homogeneous enhancement, reflecting breakdown of the blood-brain barrier during inflammatory episodes. The presence of enhancing lesions indicates ongoing disease activity and may influence treatment decisions .
Enhancement patterns can vary significantly, with some lesions showing incomplete rings (C-shaped enhancement) or nodular enhancement. The temporal evolution of enhancement, with most lesions enhancing for 4-6 weeks, helps establish the timing of inflammatory activity and can be used to demonstrate dissemination in time when combined with non-enhancing lesions.
Cerebrovascular white matter disease: small vessel ischaemia and leukoaraiosis
Cerebrovascular white matter disease represents the most common cause of white matter hyperintensities, particularly in older adults. This condition results from chronic hypoperfusion and ischaemic damage to the small penetrating arteries supplying the brain’s white matter. The term leukoaraiosis, meaning “white matter rarefaction,” describes the characteristic appearance of these changes on brain imaging.
Small vessel disease typically affects arterioles with diameters between 40-200 micrometers, leading to arterial wall thickening, luminal narrowing, and reduced cerebral blood flow. This process primarily affects watershed regions of the brain, where perfusion is most vulnerable to haemodynamic compromise. The resulting tissue changes include myelin pallor, axonal loss, and mild astrogliosis, which manifest as hyperintense signals on T2-weighted and FLAIR sequences.
The burden of cerebrovascular white matter disease correlates strongly with cardiovascular risk factors, including hypertension, diabetes mellitus, hyperlipidaemia, and smoking history.
Clinical manifestations of significant white matter disease include cognitive impairment, gait disturbances, urinary incontinence, and mood disorders. The cognitive profile typically involves executive dysfunction, reduced processing speed, and attention deficits, reflecting disruption of frontal-subcortical circuits. These symptoms often develop insidiously over years and may be mistakenly attributed to normal ageing.
Fazekas scale grading system for periventricular white matter changes
The Fazekas scale provides a standardised method for grading the severity of white matter hyperintensities, with separate assessments for periventricular and deep white matter changes. For periventricular lesions, the scale ranges from grade 0 (absent) to grade 3 (irregular periventricular signal extending into the deep white matter). Deep white matter lesions are graded from 0 (absent) to 3 (large confluent areas).
This grading system has proven valuable for research purposes and clinical correlation, with higher grades associated with increased cognitive impairment and functional decline. Fazekas grade 2 or higher is generally considered clinically significant and warrants further cardiovascular risk assessment and potential intervention.
Lacunar infarcts: small subcortical arterial territory lesions
Lacunar infarcts represent small areas of tissue necrosis resulting from occlusion of penetrating arteries, typically measuring 3-15 millimetres in diameter. These lesions appear as discrete cavitary lesions with signal characteristics similar to cerebrospinal fluid on all MRI sequences. Lacunes commonly occur in the basal ganglia, thalamus, internal capsule, and brainstem, reflecting the distribution of small penetrating vessels.
The clinical significance of lacunar infarcts depends on their location and number, with some remaining clinically silent while others cause characteristic lacunar syndromes such as pure motor hemiparesis or pure sensory stroke. The presence of multiple lacunes indicates widespread small vessel disease and increased risk of cognitive impairment and future stroke.
Binswanger’s disease: subcortical arteriosclerotic encephalopathy manifestations
Binswanger’s disease represents an extreme form of subcortical vascular dementia characterised by extensive confluent white matter changes, multiple lacunar infarcts, and cortical atrophy. This condition typically affects individuals with longstanding hypertension and diabetes, reflecting severe small vessel arteriopathy. The radiological appearance includes symmetric confluent white matter hyperintensities with relative sparing of subcortical U-fibres.
Patients with Binswanger’s disease typically present with progressive cognitive decline, gait disturbance, pseudobulbar symptoms, and urinary incontinence . The clinical course is often stepwise, with periods of stability punctuated by acute deteriorations corresponding to new vascular events.
CADASIL syndrome: hereditary cerebral arteriopathy white matter patterns
Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy (CADASIL) represents a hereditary small vessel disease caused by mutations in the NOTCH3 gene. The condition produces distinctive MRI findings, including extensive white matter hyperintensities with characteristic involvement of the temporal poles and external capsules. These locations are rarely affected in sporadic small vessel disease, making them important diagnostic markers.
CADASIL typically presents in young to middle-aged adults with migraine, recurrent strokes, cognitive decline, and psychiatric symptoms. The white matter changes often appear more extensive than would be expected from the clinical presentation, and the distinctive distribution pattern helps distinguish CADASIL from other causes of early-onset white matter disease.
Age-related white matter hyperintensities: normal ageing versus pathological changes
The prevalence of white matter hyperintensities increases dramatically with age, present in approximately 80% of individuals over 65 years and nearly universal in those over 80. These age-related changes primarily reflect cumulative effects of vascular risk factors, reduced cerebrovascular reserve, and gradual deterioration of small vessel integrity. The challenge lies in distinguishing between normal age-related changes and pathological processes requiring intervention.
Normal ageing typically produces mild, punctate periventricular hyperintensities and scattered deep white matter foci, usually measuring less than 5 millimetres in diameter. The total volume of age-related white matter changes generally remains below 10ml in healthy older adults. More extensive changes, particularly confluent lesions or rapid progression over time, suggest underlying pathological processes requiring further evaluation.
Several factors influence the development of age-related white matter changes, including genetic predisposition, cardiovascular risk factors, and lifestyle factors. Regular physical exercise, blood pressure control, and cognitive engagement may help slow the progression of age-related white matter deterioration . The clinical impact of mild age-related changes is generally minimal, though extensive changes can contribute to cognitive decline and increased fall risk.
Recent research has identified specific patterns that help distinguish normal ageing from pathological processes. Normal age-related changes tend to show symmetric distribution, gradual progression, and minimal clinical correlation, while pathological processes often demonstrate asymmetric patterns, rapid progression, or association with specific neurological symptoms. Advanced imaging techniques, including diffusion tensor imaging, can detect microstructural changes that precede conventional MRI abnormalities.
Inflammatory and autoimmune CNS conditions presenting with white matter lesions
Inflammatory and autoimmune conditions affecting the central nervous system frequently present with white matter lesions that can mimic other pathological processes. These conditions include neuromyelitis optica spectrum disorders, acute disseminated encephalomyelitis (ADEM), systemic lupus erythematosus, and various vasculitic syndromes. The distinction between these conditions and multiple sclerosis requires careful analysis of clinical presentation, imaging characteristics, and laboratory findings.
Neuromyelitis optica spectrum disorders (NMOSD) typically produce longitudinally extensive lesions affecting the spinal cord and optic nerves, with brain lesions showing distinctive patterns. Brain involvement in NMOSD often affects periventricular regions, brainstem, and areas rich in aquaporin-4 water channels. The lesions may appear more extensive and confluent than typical MS plaques, and enhancement patterns can be more persistent.
Acute disseminated encephalomyelitis presents with multifocal white matter lesions that typically appear simultaneously and show uniform enhancement patterns. Unlike MS, ADEM usually follows a monophasic course with bilateral, asymmetric lesions affecting both grey and white matter. The lesions are often larger than typical MS plaques and may show complete resolution with treatment.
Systemic autoimmune conditions can produce white matter changes through various mechanisms, including direct inflammatory infiltration, thrombotic events, or secondary effects of systemic inflammation on cerebrovascular function.
Primary angiitis of the central nervous system represents a rare but important cause of white matter lesions, characterised by inflammatory changes affecting small and medium-sized cerebral blood vessels. The imaging appearance can be highly variable, ranging from small punctate lesions to large areas of confluent signal abnormality. Conventional angiography may appear normal in small vessel involvement, making tissue diagnosis sometimes necessary for definitive diagnosis.
Differential diagnosis framework: distinguishing between white matter pathologies using advanced MRI techniques
Establishing an accurate differential diagnosis for white matter les
ions requires a systematic approach combining clinical presentation, imaging characteristics, and advanced MRI techniques. The differential diagnosis process begins with careful analysis of lesion distribution patterns, morphology, and enhancement characteristics, supplemented by clinical history and laboratory investigations.
Advanced MRI sequences provide additional diagnostic information beyond conventional T2 and FLAIR imaging. Diffusion-weighted imaging can distinguish acute from chronic lesions, while susceptibility-weighted imaging may reveal microhaemorrhages or central veins within lesions. Magnetisation transfer imaging can assess myelin integrity more specifically, and perfusion-weighted imaging can evaluate cerebrovascular hemodynamics in suspected vascular pathologies.
The presence of specific imaging features can narrow the differential diagnosis significantly. Central vein signs visible on high-resolution imaging strongly favour multiple sclerosis over vascular pathologies, while bilateral temporal pole involvement suggests CADASIL syndrome. Longitudinally extensive lesions spanning multiple vertebral segments indicate neuromyelitis optica spectrum disorders rather than typical multiple sclerosis.
Contrast enhancement patterns provide crucial temporal information about lesion activity. Ring enhancement typically indicates active demyelination, while rim enhancement may suggest ongoing inflammation. The absence of enhancement in chronic lesions helps establish disease chronicity, though some inflammatory conditions can show prolonged enhancement periods exceeding the typical 4-6 week window seen in multiple sclerosis.
The integration of clinical symptoms with imaging findings remains paramount in establishing accurate diagnoses, as identical-appearing lesions can result from vastly different pathological processes.
Quantitative MRI techniques are increasingly being incorporated into clinical practice to improve diagnostic accuracy. Lesion volume measurements, T1/T2 lesion ratios, and brain parenchymal fraction calculations can provide objective metrics for disease monitoring and treatment response assessment. Machine learning algorithms trained on large imaging datasets are beginning to assist radiologists in pattern recognition and differential diagnosis formulation.
The temporal evolution of white matter lesions provides additional diagnostic clues often overlooked in single time-point assessments. Rapidly appearing lesions suggest acute inflammatory processes or vascular events, while slowly progressive changes over months to years indicate chronic degenerative or vascular pathologies. Serial imaging at appropriate intervals can reveal characteristic progression patterns that help distinguish between different conditions causing white matter abnormalities.
Laboratory investigations complement imaging findings in establishing specific diagnoses. Cerebrospinal fluid analysis may reveal oligoclonal bands in multiple sclerosis, elevated protein in inflammatory conditions, or specific antibodies in autoimmune disorders. Serum testing for aquaporin-4 or MOG antibodies can confirm neuromyelitis optica spectrum disorders, while genetic testing may identify hereditary conditions like CADASIL or leukodystrophies.
The clinical context remains crucial in interpreting white matter lesions appropriately. Age at presentation significantly influences the differential diagnosis, with demyelinating diseases more common in younger adults and vascular pathologies predominating in older patients. The presence of systemic symptoms may suggest autoimmune or infectious etiologies, while isolated neurological symptoms might favour primary CNS pathologies.
Understanding the limitations of current imaging techniques is essential for appropriate clinical decision-making. While MRI provides excellent anatomical detail, it cannot definitively determine the underlying pathological process in many cases. Tissue biopsy may occasionally be necessary when imaging and clinical features remain inconclusive, particularly in cases where early diagnosis could significantly impact treatment decisions and long-term outcomes.
Future developments in neuroimaging continue to enhance our ability to characterise white matter pathologies more precisely. Ultra-high field MRI scanners, advanced diffusion imaging techniques, and novel contrast agents promise to provide even greater specificity in distinguishing between different causes of white matter lesions. These technological advances, combined with growing understanding of disease pathophysiology, will likely improve diagnostic accuracy and enable more personalised treatment approaches for patients presenting with white matter abnormalities on brain MRI.